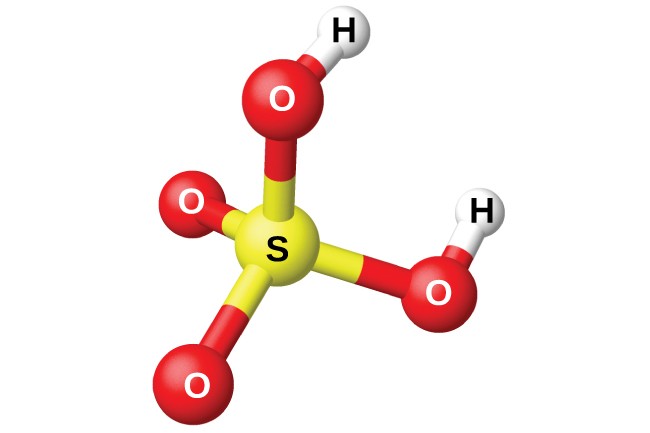
Sulfuric acid (H2SO4) – “King of chemicals” in industry
Sulfuric acid (H2SO4), is one of the most important industrial chemicals and is produced in the largest quantities in the world. Its strong acidity, hygroscopicity and strong oxidizing properties have made H2SO4 an indispensable chemical in many industries.
Why is Sulfuric acid (H2SO4) so important?
- Wide range of applications: H2SO4 is used in many industries, from fertilizer production, petroleum refining, other chemical production to paper, dye and battery production.
- Strong acidity: The ability to completely dissociate in water creates a high concentration of H+ ions, helping H2SO4 participate in many important chemical reactions.
- Hygroscopicity: H2SO4 has a very strong hygroscopicity, so it is used as a drying agent in laboratories and industry.
- Strong oxidizing properties: At high concentrations and when heated, H2SO4 exhibits strong oxidizing properties, participating in many redox reactions.
Applications of H2SO4 in industry
- Fertilizer production: H2SO4 is used to produce fertilizers such as superphosphate, ammonium sulfate.
- Petroleum refining: H2SO4 is used to remove impurities in petroleum.
- Chemical production: H2SO4 is used to produce many other chemicals such as hydrochloric acid, nitric acid, sulfate salts…
- Paper production: H2SO4 is used to treat wood, separate cellulose from lignin during paper production.
- Dye production: H2SO4 is used in the production of many dyes.
- Battery production: H2SO4 is used as an electrolyte in lead-acid batteries.
- Wastewater treatment: H2SO4 is used to adjust the pH of wastewater.

Important properties of H2SO4
- Physical properties: Viscous liquid, colorless, odorless, heavier than water, infinitely soluble in water.

- Chemical properties:
- Reaction with metals: Forms sulfate salts and hydrogen gas (except Cu, Ag, Au, Pt).
- Reaction with bases: Forms sulfate salts and water.
- Reaction with basic oxides: Forms sulfate salts and water.
- Reaction with salts: Forms new salts and new acids (if the new acid is weaker than H2SO4).
- Hydration: Absorbs water strongly, carbonizes sugar and paper.
- Oxidation: At high concentrations and when heated, H2SO4 exhibits strong oxidizing properties.

Safety when using H2SO4
H2SO4 is a dangerous chemical that can cause severe burns when in contact with the skin, eyes and respiratory tract. Therefore, when working with H2SO4, it is necessary to comply with chemical safety regulations:
- Labor protection: Use gloves, goggles, and masks when working with H2SO4.
- Storage: Store H2SO4 in a sealed container, in a dry, cool place, away from heat sources and flammable substances.
- Transportation: Transport H2SO4 by specialized means, ensuring safety.
- In case of an accident: If H2SO4 accidentally splashes on you, quickly rinse with clean water and take to the nearest medical facility.
Conclusion
H2SO4 is an important chemical with many industrial applications. However, the use of H2SO4 requires strict compliance with safety regulations to ensure worker safety and environmental protection.
Vina TS is a reputable unit specializing in providing industrial chemicals in the Vietnamese market. If you want to buy H2SO4, please contact us via the following information:
Address: 82 Vu Tong Phan, An Phu Ward, Thu Duc City, Ho Chi Minh City
Hotline: 098 777 0438 (Mr. Tan)/ 0949446009 (Ms. Chau)
E-mail: vinatsinfo@gmail.com